Nanovesicle-related technology
Extracellular nanovesicles are membrane "bubbles" about 100 nm in size, secreted by cells into the extracellular space and biological fluids. The nanovesicles composition reflects but not repeats the composition of the cells that produce them. This phenomenon defines the high diagnostic potency of extracellular nanovesicles circulating in the blood and other biological fluids. Moreover, the circulating nanovesicles are supposed to form a natural system of cell-to-cell communication and to present potential vehicles for drug delivery.
PROJECTS::
- Immuno-sorption and quantitative analysis of the specific tissue-derived nanovesicles
- Development and validation of new ligands (DNA aptamers) for tissue-specific nanovesicles
- Quantitative analysis of specific nanovesicles using nano-enzymes
STANDARD METHODS / EQUIPMENT:
- Ultracentrifugation (Optima ™ XE / Beckman Coulter Life Sciences)
- Correlation spectroscopy (Nanotrac Wave II, manufactured by Microtrac)
- Analysis of trajectories of nanoparticles (NanoSight NS300 with highly sensitive sCMOS camera)
NEW TECHNOLOGIES:
- Isolation of nanovesicles from plasma using a composite polymer system (Patent (№ 2741776 dated 25.02.2020)
- Isolation of nanovesicles by affinity sorption on superparamagnetic particles (SPMP) and quantitative analysis by flow cytometry (Patent № 2741638 dated 11.03.2020)
- Use of nanoparticles enzyme-mimetic properties for quantitative analysis of specific vesicular fractions
FUNDING: Research is being carried out within the framework of Ministry of Health Assignment No. 056-00108-21-00 dated December 23 2020 as a research project: "Development and clinical testing of methods for isolation, analysis and modification of the circulating plasma nanovesicles for the purpose of personalized selection and optimization of the standard approaches of anticancer systemic therapy.”
IMMUNO-SORPTION AND QUANTITATIVE ANALYSIS OF THE SPECIFIC TISSUE-DERIVED NANOVESICLES
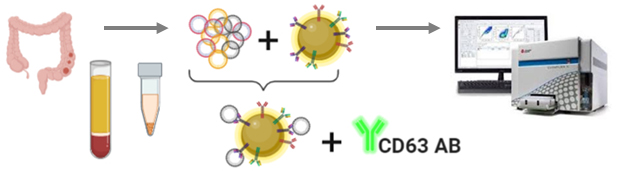

Development of technology for quantitative analysis of tissue-specific population of plasma nanovesicles in order to diagnose and monitor colorectal cancer therapy. The study involves search and validation of protein markers of intestinal epithelium differentiation, the creation of a method for immune sorption of nanovesicles using antibodies and superparamagnetic particles (SPMP), and quantitative analysis of the isolated nanovesicles using flow cytometry.
The study is carried out in cooperation with the Department of Chemotherapy
Investigator:Nazarova Inga
DEVELOPMENT AND VALIDATION OF NEW LIGANDS (DNA APTAMERS) FOR TISSUE-SPECIFIC NANOVESICLES
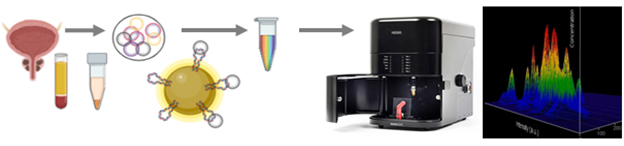

Development of technology for the tissue-specific nanovesicles isolation from plasma using DNA aptamers and superparamagnetic particles (SPMP) in order to diagnose oncological diseases (prostate cancer, lung cancer). The research involves application of previously described aptamers and searches for new molecules using SELEX technology (Systematic Evolution of Ligands by EXponential Enrichment)
The study is carried out in cooperation with the Department of Oncourology
Investigator: Nikiforova Nadezhda
QUANTITATIVE ANALYSIS OF SPECIFIC NANOVESICLES USING NANO-ENZYMES
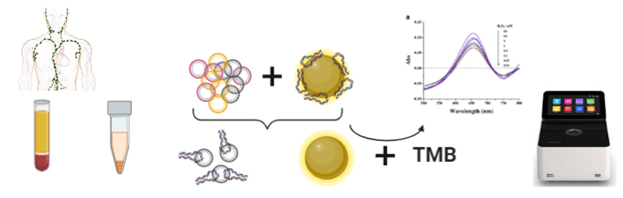

Creation of a method for quantitative analysis of specific nanovesicles in plasma using enzyme-mimetic properties of nanoparticles (iron oxide, gold). This technology will be applied for monitoring of lymphoproliferative diseases during course of chemotherapy.
The study is carried out in cooperation with the Department of Chemotherapy
Investigator: M. Slyusarenko
HEAD OF THE LAB:

RESEACH STAFF:
“Onco-endocrinology” team
Berstein L.M., MD., prof.
Tsyrlina E.V., MD., PhD.
Vasiliev D.A., MD., PhD.
Kovalenko I.M., MD., PhD
“Nanovesicles-related technology” team
Nazarova I.V.
Nikifiriva N.S.
Slyusarenko M.A.
“Methods of miRNA analysis” team:
Sidina E.I.
Knyazeva M.S.
Zabegina L.M.
Malysheva S.A.
